Introduction
Peptides are widely used in research and laboratory settings, requiring proper reconstitution before use. Reconstitution is the process of dissolving peptide powder into a liquid solvent to achieve the desired concentration. This guide outlines the necessary materials, storage considerations, unit conversions, and a step-by-step approach to reconstitute peptides while preventing contamination properly. Accurate data is crucial in peptide reconstitution to ensure reliable experimental results. Learn how to reconstitute peptides, utilize solvents, and use proper handling techniques.
Understanding Peptide Storage
Properly store peptides to maintain their integrity and prevent degradation caused by external factors such as heat, light, and pH conditions. Lyophilized (freeze-dried) peptides are highly stable when stored in a cool, dry environment, away from direct light and moisture. The best storage practices include:
- Short-Term Storage: Keep peptides at 2–8°C (refrigerated) if they will be used within a few weeks.
- Long-Term Storage: Store at -20°C (freezer) for extended stability.
- After Reconstitution, peptides should be kept refrigerated and used within a specified timeframe to prevent degradation.
Tip: Always allow peptides to reach room temperature before opening vials to prevent condensation, which can introduce moisture.
Understanding Peptide Reconstitution
What is Peptide Reconstitution?
Peptide reconstitution is the process of dissolving lyophilized or powdered peptides into a solvent to restore them to their original, active form. This step is crucial for experimental procedures and peptide-based research. Accurate reconstitution ensures that peptides retain their biological activity and functionality. Incorrect reconstitution, on the other hand, can lead to peptide degradation, reduced activity, or inaccurate measurements, which can compromise the integrity of your research or application. By understanding the importance of precise measurements and the proper techniques, you can enhance the quality and reliability of your peptide solutions.
Peptide Stability and Storage
Proper storage of reconstituted peptides is vital for maintaining their stability and preventing degradation for reliable research outcomes. Typically, peptides should be stored at -20°C or -80°C to prevent degradation. It’s important to avoid repeated freeze-thaw cycles, as these can adversely affect peptide stability. For short-term storage, keeping peptides at 4°C is acceptable, but they should be used promptly to avoid potential degradation. Handling peptides carefully to avoid environmental stress, such as exposure to light and moisture, is also essential to protect their quality and functionality.
Materials Needed for Reconstitution
Before starting the reconstitution process, ensure you have the following materials:
- Lyophilized peptide (in powder form)
- Bacteriostatic water (BAC water) or sterile water
- Alcohol wipes (for sanitation)
- Sterile insulin or precision syringe (1 mL or 3 mL recommended)
- Sterile needle (18-22 gauge for drawing, 30 gauge for smaller applications)
- Sterile vial for storage (if necessary)
- Preparation of a stock solution: Choose the proper solvent based on the peptide’s hydrophobic or hydrophilic nature. Dissolve peptides in pure solvents before further dilution in assay buffers.
Selecting the Right Solvent
The choice of solvent is critical for effective peptide reconstitution, as improper solvents can lead to incomplete dissolution or degradation. Peptides generally fall into two categories: hydrophilic peptides, which dissolve easily in sterile water or bacteriostatic water, and hydrophobic peptides, which may require organic solvents like DMSO or acetic acid before dilution in a buffer. Additionally, certain peptides are pH-sensitive and may require specific buffers (e.g., phosphate-buffered saline or ammonium bicarbonate) to maintain stability. Always check solubility guidelines for each peptide before selecting a solvent.
What Is Bacteriostatic Water?
Bacteriostatic water (BAC water) is a sterile, preservative-containing solvent used to dissolve peptides. Unlike sterile water, which lacks preservatives and is best used immediately, BAC water contains 0.9% benzyl alcohol, which helps inhibit bacterial growth and extends the shelf life of reconstituted peptides.
Key Differences Between BAC Water and Sterile Water:
| Solvent Type | Preservative | Shelf Life After Reconstitution | Common Use Cases |
|---|---|---|---|
| Bacteriostatic Water | 0.9% benzyl alcohol | Up to 28 days | Multi-use vials |
| Sterile Water | None | 24 hours | Single-use applications |
For research applications requiring multiple withdrawals from a vial, BAC water is typically preferred to reduce contamination risks.
Preventing Contamination
To ensure sterility and prevent bacterial contamination:
🧼Sanitize: Clean all surfaces, hands, and materials with alcohol wipes before handling peptides.
🧴 Use sterile equipment: Always use new, sterile syringes and needles.
🚫 Avoid cross-contamination: Never touch syringe tips or vial openings.
🌡️ Minimize exposure: Reconstitute peptides quickly and return them to cold storage immediately.
Understanding Unit Conversions
Accurate calculations are essential to achieving the correct peptide concentration. Below is a guide to common unit conversions:
- 1 milligram (mg) = 1,000 micrograms (mcg)
- 1 milliliter (mL) = 1 cubic centimeter (cc) = 1,000 microliters (µL)
- Volume (liquid) vs. Weight (powder): Peptides are measured in milligrams (mg), while liquid solvents are measured in milliliters (mL).
Example Calculation:
If you dissolve 5mg of peptide in 2mL of BAC water, the concentration would be:
2 mL 5000 mcg=2500 mcg/mL
Each 1mL of solution contains 2500 mcg (or 2.5 mg) of peptide, making accurate measurements easier.
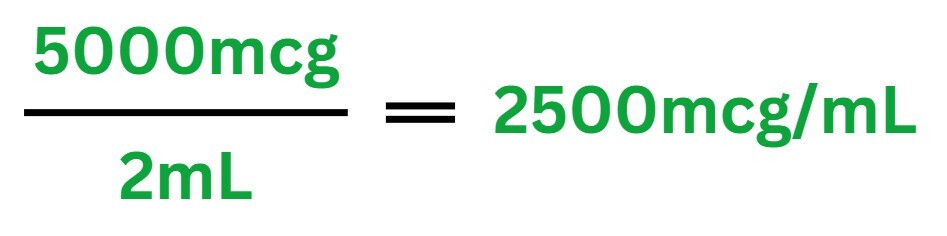
Step-by-Step Guide to Reconstituting Peptides
Step 1: Sanitize Your Work Area
- Clean your hands with soap or sanitizer.
- Wipe down surfaces, vials, and syringe packaging with alcohol wipes.
Step 2: Prepare the Syringe and Solvent
- Use a sterile syringe to withdraw the desired amount of bacteriostatic water.
- Tip: Use an 18-22 gauge needle for easier solvent withdrawal.
Step 3: Insert Solvent into Peptide Vial
- Remove the cap from the peptide vial and clean the rubber stopper with an alcohol wipe.
- Insert the syringe needle into the vial at a slight angle to prevent excessive pressure buildup.
- Slowly inject the solvent against the side of the vial (not directly onto the peptide powder) to prevent foaming or denaturation.
Step 4: Allow the Peptide to Dissolve
- DO NOT SHAKE the vial, as this can damage the peptide structure.
- Gently swirl or rotate the vial until the peptide is fully dissolved.
- If necessary, let the vial sit at room temperature for a few minutes to facilitate dissolution.
Step 5: Store the Reconstituted Peptide
- Label the vial with the peptide name, concentration, and reconstitution date.
- Store in the refrigerator (2-8°C) and avoid repeated freeze-thaw cycles.
Final Thoughts
Proper peptide reconstitution ensures stability, accuracy, and sterility in research applications. By following best practices for storage, handling, and unit conversions, you can effectively reconstitute peptides while minimizing contamination risks. Always adhere to laboratory safety guidelines and use sterile materials throughout the process.
References:
1. Ferrazzano, L., Catani, M., Cavazzini, A., Martelli, G., Corbisiero, D., Cantelmi, P., … & Tolomelli, A. (2022). Sustainability in peptide chemistry: current synthesis and purification technologies and future challenges. Green Chemistry, 24(3), 975-1020.
2. Wright, P. E., & Dyson, H. J. (2015). Intrinsically disordered proteins in cellular signaling and regulation. Nature Reviews Molecular Cell Biology, 16(1), 18-29.
3. Wilkinson, A. S., Allwood, M. C., Caspersen, V., Finnis, R., Miller, J., Wallace, A., … & Lawrence, S. (2016). A novel study demonstrating practical shelf life extension (Beyond Use Dating) of. Young, 30, 04.
4. Piascik, P. (2016). Dispensing biotechnology products: handling, professional education, and product information. In Pharmaceutical Biotechnology (pp. 445-454). CRC Press.
5. Lai, M. C., & Topp, E. M. (1999). Solid‐state chemical stability of proteins and peptides. Journal of Pharmaceutical Sciences, 88(5), 489-500.

